Looking back: Our COVID-19 vaccine trial at 1 year
Dr. Rita Mangione-Smith reflects on the year since KPWHRI launched the world's first clinical trial for a COVID-19 vaccine
By Rita Mangione-Smith, MD, MPH, vice president for research and health care innovation at Kaiser Permanente Washington, executive director and senior investigator of Kaiser Permanente Washington Health Research Institute, professor of health systems science at Kaiser Permanente School of Medicine
One year ago — on March 16, 2020 — KPWHRI gave the first-ever dose of a COVID-19 vaccine candidate to a volunteer from our community. Jennifer Haller received the first injection of the Moderna investigational vaccine for COVID-19 as part of our phase 1 clinical trial led by Senior Investigator Lisa A. Jackson, MD, MPH. As of mid-February, 25.5 million doses of this vaccine had been administered in the United States, including to many Kaiser Permanente members.
Looking back, I'm grateful to our trial participants and all research volunteers who helped test this vaccine and others. It takes courage to test an unapproved vaccine, and without their willingness to be “the first,” the world would not have this crucial resource against the coronavirus. I acknowledge the amazing scientists at Moderna and the National Institutes of Health who created this new mRNA vaccine and developed it so rapidly that it was awarded Emergency Use Authorization by the U.S. Food and Drug Administration only 9 months since that first injection.
I want to express gratitude for our hardworking researchers, especially in our clinical research program. They've made a tremendous contribution to public health with their vaccine studies. Many have been working onsite at KPWHRI facilities throughout the pandemic. And our vaccination success is possible because of Kaiser Permanente leadership, including in our Washington region, and extra hours by Kaiser Permanente employees to give vaccines to members while continuing to provide excellent care as usual.
Our COVID-19 work continues. Vaccine trials are ongoing, and we anticipate new studies on viral variants. We're planning to start studying vaccines for youth and children, too. Finally, our vaccine surveillance researchers are now monitoring the vaccines to ensure their safety and effectiveness as people across the country receive them.
I'm hopeful because all 3 of the vaccines now available in the United States appear safe and effective. Recent national guidelines say that after we're fully vaccinated, we can safely gather with others who are vaccinated, even indoors without masks or distancing. For now, please continue to follow guidelines to stay safe and join me in expressing gratitude to all who made our new vaccines possible, especially our clinical trial volunteers.
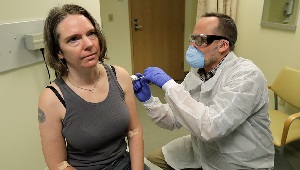 COVID-19 vaccine trial volunteer on the shot heard 'round the world
COVID-19 vaccine trial volunteer on the shot heard 'round the world
On a visit to KPWHRI a year ago, Jennifer Haller became the first person ever to be injected with a COVID-19 vaccine in a clinical trial.
 ‘Fighting back’ against COVID-19: Remembering a historic clinical trial
‘Fighting back’ against COVID-19: Remembering a historic clinical trial
Dr. Lisa Jackson, KPWHRI senior investigator, recounts the genesis of a groundbreaking vaccine.
 Unvaccinated volunteers needed for COVID-19 booster trial
Unvaccinated volunteers needed for COVID-19 booster trial
NIAID-sponsored study includes Seattle in test for benefits of mixing different vaccine types.
More COvid-19 news
IN THE MEDIA
-
FDA authorizes a second coronavirus vaccine, a turning point in the pandemic (Washington Post,
Dec. 18, 2020) -
Luck, foresight, and science: How an unheralded team developed a COVID-19 vaccine in record time
(USA Today, Jan. 26, 2021) -
Opinion: I got vaccinated to support my community (South Seattle Emerald, Feb. 22, 2021)
-
What we know about the Johnson & Johnson COVID-19 vaccine: Timing, dosage, access in Washington state (Seattle Times, Feb. 26, 2021)
-
The women behind the vaccine: Meet the scientists leading the fight to end the pandemic (People Magazine, March 4, 2021)
Suggestions?
Please send comments or suggestions to Communications Director Caroline Liou at Caroline.X.Liou@kp.org


