ACT Study contributes to understanding Alzheimer’s disease in brain cells
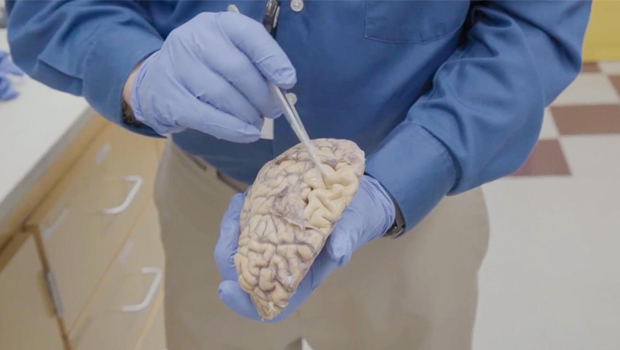
UW Medicine pathologist C. Dirk Keene, MD, points out large grooves in the surface of a brain of someone who died from Alzheimer’s disease and donated their brain to research. In a healthy brain, these folds would nestle right up against each other but in Alzheimer’s, neuron death leads to noticeable shrinkage in many parts of the brain. Keene, a KPWHRI affiliate investigator, is part of a collaborative effort to find the cellular roots of Alzheimer’s. Photo by Erik Dinnel/Allen Institute
Mapping the disease at the cellular level identifies possible new treatment targets
Researchers from the Allen Institute for Brain Science, Kaiser Permanente Washington Health Research Institute (KPWHRI), and UW Medicine have created the most detailed picture yet of how Alzheimer’s disease progresses at the cellular level.
"This work advances our understanding of how the brain changes when Alzheimer's disease starts and as it advances," said KPWHRI Principal Collaborative Scientist Nicole Gatto, PhD, MPH, an author on the study. Other KPWHRI coauthors were Project Managers Lynn Fleckenstein and Kelly Meyers.
Using data and samples from volunteers, including Kaiser Permanente Washington members participating in the Adult Changes in Thought Study (ACT Study), the researchers used advanced genomic technologies and machine learning models to create a timeline of the cellular and molecular changes caused by the disease. In the process, they found that a type of inhibitory neuron (the somatostatin-expressing inhibitory neuron) is one of the earliest cell types that is lost in Alzheimer's disease — a surprising discovery that could highlight potential targets for future therapies.
The study, published in Nature Neuroscience, analyzed over 3.4 million cells from 84 donated brains, most from volunteers in the ACT Study. Many ACT Study participants, who may or may not develop dementia, agree to donate their brain to research after their death. By making this massive dataset available through the Seattle Alzheimer’s Disease Brain Cell Atlas (SEA-AD) consortium, supported through the National Institute on Aging, the scientists hope to accelerate global research.
Creating a pathology clock
The study zoomed in on a region of the brain cortex called the middle temporal gyrus that is involved in language, memory, and higher-order visual processing. This region is also a critical zone where preclinical Alzheimer’s pathology, like the buildup of toxic protein fragments, transitions to more advanced neurodegeneration linked to dementia.
The new knowledge provided by this study may help scientists and drug developers around the world develop diagnostics and treatments targeted to specific stages of Alzheimer’s and other causes of dementia.
To explore this progression, the researchers harnessed single-cell and spatial genomics technologies developed with funding by the National Institutes of Health’s BRAIN Initiative Cell Census Network. They used these tools to map the active genes, DNA structure, and precise location of individual cells in the middle temporal gyrus region in brain samples from people with Alzheimer's disease. They compared the results to a normal brain reference map. Machine learning and other advanced methods were used to construct a high-resolution view of how the disease impacts different cell populations over time.
The researchers identified 2 distinct phases of Alzheimer's disease: a slow, early buildup of abnormal cellular changes that occurs before any memory or cognitive impairments appear, followed by a later rapid increase that coincides with cognitive decline.
The data suggests a narrative for how the disease unfolds: Changes in the brain’s immune and support cells promote, or dysregulate, inflammation leading to the early loss of specific neurons that regulate the balance of excitation and inhibition in the cortex.
"The ACT Study and our partners are contributing to better understanding of Alzheimer's disease and other dementias at multiple levels, from preventive behaviors to molecular targets of new therapies," said KPWHRI Senior Investigator Linda McEvoy, PhD, lead investigator of the ACT Study. "This work is possible because of Act Study volunteers and others who volunteer to participate."
Research reported in this publication was supported by the National Institute on Aging, which is part of the National Institutes of Health (U19AG060909, P30AG066509, U19AG066567). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
This has been adapted from a news story by Jake Siegel at the Allen Institute.
Learn About the ACT Study

Understanding brain aging
For over 30 years, the Adult Changes in Thought (ACT) Study has been advancing our understanding of cognition, aging, and better ways to delay and prevent Alzheimer’s disease and related dementias.
News

New open data to help understand Alzheimer’s
Cell by cell, scientists are building a high-resolution map of brain changes in Alzheimer’s disease.
News

Seattle team to build brain map of Alzheimer’s disease
$40.5M NIH-funded project aims to pinpoint cells at root of brain disease—and new therapeutic targets.


