Long-term opioid risks are the focus of $10 million GHRI study
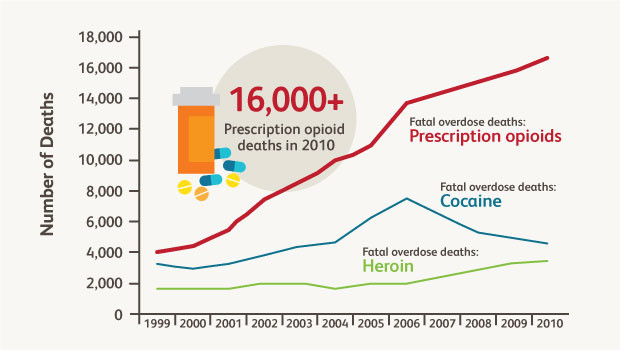
Trends in U.S. drug overdose deaths. Adapted from Okie S. N Engl J Med. 2010 363:1981-1985.
Dr. Denise Boudreau launches a collaborative project with Kaiser and other health systems on the safety of long-term prescription opioid use.
Prescription opioid abuse has "a devastating impact on public health and safety," according to the White House Office of National Drug Control Policy. Fatal overdoses of prescription opioids such oxycodone quadrupled since 1999. This trend may be noticeably affecting the lifespan of some groups of Americans.
"We don't have an accurate measure of the true magnitude of the problem," says Group Health Research Institute (GHRI) Senior Investigator Denise Boudreau, PhD. "Opioid misuse, abuse, and addiction are likely underreported in medical records, so opioid use disorder may be even more common than current estimates." In response, the U.S. Food and Drug Administration (FDA) mandated a series of safety studies on prescription opioids. Group Health and GHRI are involved in three.
Leaders in reducing opioid harms
Group Health and GHRI are innovators in practical approaches to safer opioid therapy. For example, a 2015 study by GHRI Senior Investigator Michael Von Korff, ScD, and colleagues showed that a Group Health program plus new state guidelines worked better than guidelines alone at reducing high doses for people on chronic opioid therapy.
Based in part on this track record, GHRI will receive $10 million for one of the largest of the FDA-mandated opioid studies, led by Dr. Boudreau and Carla A. Green, PhD, MPH, Kaiser Permanente Center for Health Research, which is part of Kaiser Permanente Northwest. The researchers will measure the risk of misuse, abuse, and addiction associated with long-term use of extended-release/long-acting (ER/LA) opioids for chronic noncancer pain. The project will also investigate factors that influence that risk.
As directed by the FDA mandate, funding comes from pharmaceutical companies that manufacture ER/LA opioids. "The FDA, the pharmaceutical industry, and health care leaders agree that we have gaps in our knowledge about opioids," Dr. Boudreau says. "We need better data to quantify the magnitude of risk and understand what makes some people more susceptible than others to the potential harms of opioids."
Dr. Boudreau's Medication Use, Safety, and Evidence (MUSE) study will help fill these information gaps. To ensure that the results reflect the entire United States, MUSE takes advantage of GHRI's extensive research network to achieve a diverse study population. Study participants and data are from:
- Group Health, Kaiser Permanente Northwest, Kaiser Permanente Southern California, Henry Ford Health System, Geisinger Health System, and Meyer’s Primary Care Institute—all part of the Health Care Systems Research Network.
- Veterans Affairs, Palo Alto, CA;
- New York City Research and Improvement Networking Group sponsored by the Albert Einstein College of Medicine Department of Family & Social Medicine, and University of Florida, Gainsville, of the national Practice-Based Research Network.
Data for MUSE will come from electronic health records, insurance claims, and interviews and surveys with study participants.
Two studies in one
MUSE has two parts:
- To estimate potential harms (misuse, abuse, and addiction) associated with long-term opioid therapy, the researchers will do a cross-sectional study of patients on chronic opioid therapy including ER/LA opioids for at least one year.
- To understand the rate of developing misuse, abuse, and addiction associated with long-term use of ER/LA use for chronic pain, the researchers will do a prospective study of people who recently started using the drugs. The study will look at factors including age, sex, race/ethnicity, type of medication, behaviors such as alcohol use, stress, personal genetics, and reason for the prescription, to see if they influence patient outcomes.
The MUSE study is currently doing pilot work on the process of recruiting potential study participants and collecting data from the nine sites. In early 2017, researchers will begin recruiting patients for the full study. The FDA will receive a final report in 2020.
In other FDA-mandated opioid work at GHRI, David Carrell, PhD, clinical data scientist, and Eric Johnson, MS, biostatistician, are developing algorithms to apply in diverse health care settings to detect opioid abuse or addiction using data from medical visits.
Jennifer Nelson, PhD, senior investigator and director of biostatistics, and Susan Shortreed, PhD, associate investigator, co-lead a Statistical Coordinating Center for projects, including Dr. Carrell’s, investigating ER/LA opioid-related overdoses and deaths. Andrea Cook, PhD, GHRI associate investigator; Onchee Yu, MS, senior biostatistician; and biostatisticians Eric Johnson and Rob Wellman, MS, will contribute to this work, which is part of overall data coordination by Mary Ann McBurnie, PhD, Kaiser Permanente Center for Health Research.
"Partnering with the FDA to understand the benefits, risks, and safety of medicines is part of our mission of protecting patients and providing them with the best care,” Dr. Boudreau says. "Our studies will determine who has the greatest risks from ER/LA opioids so we can improve prescription opioid monitoring and guidelines."
by Chris Tachibana




