Kaiser Permanente launches first coronavirus vaccine trial
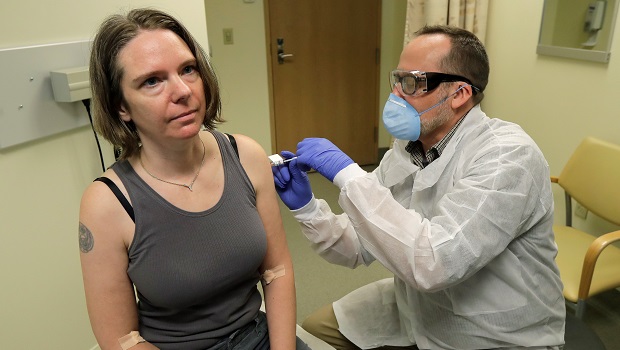
Jennifer Haller, a clinical trial volunteer, receives the first-ever injection of an investigational vaccine for the coronavirus. Credit: Ted S. Warren / AP Photos
NIH-funded trial of Moderna mRNA vaccine is first of any for COVID-19
THIS STORY WAS UPDATED FROM PREVIOUS VERSIONS PUBLISHED MARCH 4, MARCH 16, AND MARCH 19, 2020.
On March 16, Kaiser Permanente Washington Health Research Institute (KPWHRI) gave the first-ever injection of an investigational vaccine for the 2019 novel coronavirus, SARS-CoV-2, to 4 volunteers participating in a phase 1 federally sponsored clinical trial.
As of March 16, no other trial had been launched in people of any vaccine for this virus, which causes COVID-19. The trial at KPWHRI’s Seattle-based Vaccine Treatment and Evaluation Unit (VTEU) began recruiting participants on March 3. “We are proud that the National Institute of Allergy and Infectious Diseases (NIAID) selected us to conduct this innovative trial,” said Lisa Jackson, MD, MPH, senior investigator at KPWHRI. “We’re well prepared and focused on helping to address this evolving health situation.” Dr. Jackson is the lead researcher for the study, funded by the U.S. National Institutes of Health.
“Finding a safe and effective vaccine to prevent infection with SARS-CoV-2 is an urgent public health priority,” said NIAID Director Anthony S. Fauci, MD, in a NIAID news release. “This phase 1 study, launched in record speed, is an important first step toward achieving that goal.”
Emory University’s VTEU in Atlanta, Georgia joined the trial on March 27. NIAID added the second site to ensure that the trial can move forward without dependence on a single geographic location.
The investigational vaccine is called mRNA-1273 and made by Moderna. The vaccine is made using a new process that is much faster than older methods of making vaccines. It does not contain any part of the actual coronavirus and cannot cause infection. Instead, it includes a short segment of messenger RNA that is made in a lab.
The trial will involve 45 healthy adults
Study participants must be healthy adults age 18 to 55 years. To be eligible, they can’t have certain health conditions that affect the immune system, and they can’t be taking medications that affect the immune system.
The initial trial will involve just 45 participants—28 at KPWHRI and 17 at Emory University. It is a “phase I” test of a 3-phase process examining the potential vaccine. In this first phase, researchers are testing the safety of various doses and whether these doses produce an immune response. Phase I trials don’t study the effectiveness of the vaccine in preventing coronavirus infection. That work comes at a later phase of the vaccine research.
Response to study recruitment efforts has been very positive, so the study teams no longer need to identify potential study participants. Online study recruitment is now closed.
KPWHRI’s path to this important role
KPWHRI’s vaccine research team began preparations for the possibility of a trial as soon as they got the call on January 28. The team has expertise in conducting these kinds of trials, including testing other investigational vaccines against “swine” and “bird” flu. KPWHRI became a VTEU site in 2007, and it is the only one of the nation’s 9 VTEU centers not housed at a university medical center. Since 1962, the VTEUs have played a key role in developing new and improved vaccines and therapies against infectious diseases.
Currently there is no vaccine proven to protect against SARS-CoV-2 infection. A vaccine is urgently needed for several important reasons:
- The large number of people infected
- The ability of the virus to spread from person to person
- The spread of the virus across so many geographic areas
By Rebecca Hughes
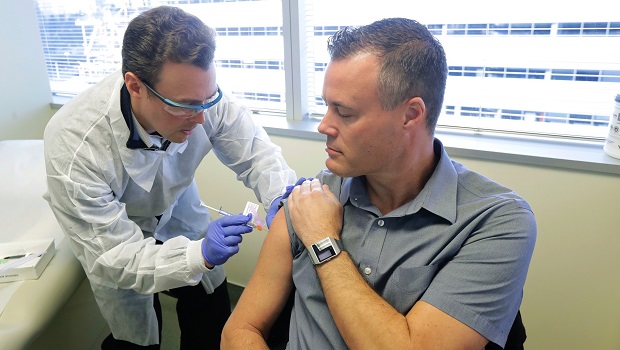
Neal Browning becomes the second volunteer to receive the investigational COVID-19 vaccine in the NIH-supported clinical trial in Seattle. Credit: Ted S. Warren / AP Photos
research

Clinical trial of H7N9 bird flu vaccine starts at KPWHRI
Dr. Lisa A. Jackson leads national trial to explore improving immune responses to the vaccine.
Read it in News and Events.



