Brian Williamson, PhD
Biography
Brian Williamson, PhD, is a biostatistician with expertise in statistical epidemiology, semiparametric and nonparametric estimation theory, and high-dimensional estimation and prediction. He is interested in developing robust procedures for statistical inference when machine learning is used to address problems in public health, and in working toward accessible, affordable, high-quality healthcare for everyone. A central theme of his research is using prediction models (including machine learning and artificial intelligence) to make more accurate and efficient use of electronic health records data for research and clinical care.
Before joining Kaiser Permanente Washington Health Research Institute, Dr. Williamson completed his postdoctoral research training at the Fred Hutchinson Cancer Research Center. During his time at Fred Hutch, Dr. Williamson developed statistical methods to address issues arising in the development of biomarker panels for use in risk prediction, screening, and diagnosis. Dr. Williamson also collaborated with researchers from the Women’s Health Initiative to assess the utility of metabolomic biomarkers for predicting breast and colorectal cancer; with researchers from the HIV Vaccine Trials Network (HVTN) to aid in selecting candidate broadly neutralizing antibody regimens to advance to HIV prevention clinical trials; and was a part of the Coronavirus Prevention Network Biostatistics Team.
Dr. Williamson received his PhD in biostatistics from the University of Washington. His dissertation focused on a general framework for performing inference on model-free variable importance measures. With colleagues from the HVTN, he used this framework to identify features of the HIV viral genome that may be important in predicting viral susceptibility to the broadly neutralizing antibody VRC01.
At KPWHRI, Dr. Williamson collaborates on projects across a range of research areas including mental health, pragmatic clinical trials, and drug and vaccine safety and effectiveness.
Recent Publications
Balderson BH, Gray SL, Fujii MM, Nakata KG, Williamson BD, Cook AJ, Wellman R, Theis MK, Lewis CC, Key D, Phelan EA A health-system-embedded deprescribing intervention targeting patients and providers to prevent falls in older adults (STOP-FALLS trial): study protocol for a pragmatic cluster-randomized controlled trial 2023 May 11;24(1):322. doi: 10.1186/s13063-023-07336-7. Epub 2023-05-11. PubMed
Benkeser D, Montefiori DC, McDermott AB, Fong Y, Janes HE, Deng W, Zhou H, Houchens CR, Martins K, Jayashankar L, Castellino F, Flach B, Lin BC, O'Connell S, McDanal C, Eaton A, Sarzotti-Kelsoe M, Lu Y, Yu C, Borate B, van der Laan LWP, Hejazi NS, Kenny A, Carone M, Williamson BD, Garver J, Altonen E, Rudge T, Huynh C, Miller J, El Sahly HM, Baden LR, Frey S, Malkin E, Spector SA, Andrasik MP, Kublin JG, Corey L, Neuzil KM, Carpp LN, Pajon R, Follmann D, Donis RO, Koup RA, Gilbert PB, Immune Assays, Moderna Inc., Coronavirus Vaccine Prevention Network (CoVPN)/Coronavirus Efficacy (COVE), United States Government (USG)/CoVPN Biostatistics Teams Comparing antibody assays as correlates of protection against COVID-19 in the COVE mRNA-1273 vaccine efficacy trial 2023 Apr 19;15(692):eade9078. doi: 10.1126/scitranslmed.ade9078. Epub 2023-04-19. PubMed
Huang Y, Zhang Y, Seaton KE, De Rosa S, Heptinstall J, Carpp LN, Randhawa AK, McKinnon LR, McLaren P, Viegas E, Gray GE, Churchyard G, Buchbinder SP, Edupuganti S, Bekker LG, Keefer MC, Hosseinipour MC, Goepfert PA, Cohen KW, Williamson BD, McElrath MJ, Tomaras GD, Thakar J, Kobie JJ, NIAID HIV Vaccine Trials Network (HVTN) 073/South African AIDS Vaccine Initiative (SAAVI) 102, HVTN 086/SAAVI 103, HVTN 094, HVTN 097, HVTN 098, HVTN 100, HVTN 105, HVTN 111, and HVTN 205 Protocol Leadership Teams Baseline host determinants of robust human HIV-1 vaccine-induced immune responses: A meta-analysis of 26 vaccine regimens 2022 Oct;84:104271. doi: 10.1016/j.ebiom.2022.104271. Epub 2022-09-27. PubMed
Hughes JP, Williamson BD, Krakauer C, Chau G, Ortiz B, Wakefield J, Hendrix C, Amico KR, Holtz TH, Bekker LG, Grant R Combining information to estimate adherence in studies of pre-exposure prophylaxis for HIV prevention: Application to HPTN 067 2022 Mar 15;41(6):1120-1136. doi: 10.1002/sim.9321. Epub 2022-01-25. PubMed
Huang Y, Williamson BD, Moodie Z, Carpp LN, Chambonneau L, DiazGranados CA, Gilbert PB. Analysis of neutralizing antibodies as a correlate of instantaneous risk of hospitalized dengue in placebo recipients of dengue vaccine efficacy trials. J Infect Dis. 2021 Jun 26:jiab342. doi: 10.1093/infdis/jiab342. Online ahead of print. PubMed
News
KPWHRI begins new phase of flu surveillance
KPWHRI receives $10 million to continue vaccine effectiveness research for flu, COVID-19, and other respiratory diseases.
Vaccine Safety

Biostatisticians track COVID-19 vaccine safety
Dr. Jennifer Nelson explains how KP scientists are helping the CDC and FDA keep an eye out for rare adverse events.
mental health
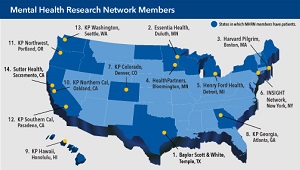
$10 million to expand Mental Health Research Network
NIMH funding will enable the MHRN to conduct larger studies in integrated health systems on topics that matter most.



