COVID-19 variant vaccine trial begins at KPWHRI
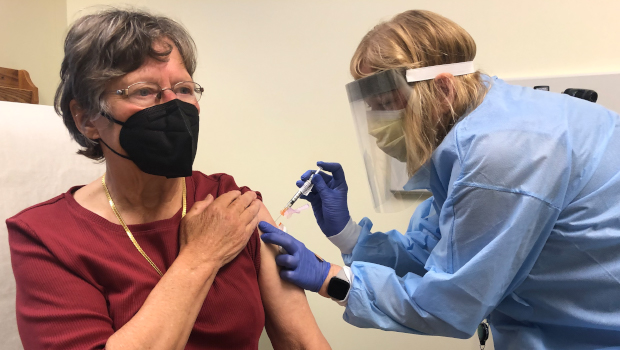
As part of a phase 1 clinical trial of a variant-specific COVID-19 vaccine developed by Moderna, pharmacist Sue Lasicka gives a shot of the vaccine candidate to volunteer Suzanne Uvelli-Spencer, MD, on April 1, 2021, at Kaiser Permanente Washington Health Research Institute in Seattle.
An investigational vaccine developed by Moderna is being tested for safety and immune response in Seattle and 3 other cities
An investigational vaccine designed to protect against the coronavirus variant first detected in the Republic of South Africa was administered April 1 to 3 adult volunteers enrolled in a new phase 1 clinical trial at Kaiser Permanente Washington Health Research Institute (KPWHRI). The trial was officially launched the day before with shots being given at another study site in Atlanta.
The vaccine was developed by the biotechnology firm Moderna Inc., based in Cambridge, Massachusetts. Led and funded by the National Institute of Allergy and Infectious Diseases (NIAID) of the National Institutes of Health, the trial is testing the immune response generated by the new vaccine candidate as well as its safety. The trial will not determine whether the vaccine prevents infection by the new variant or stops its spread in the population.
Researchers aim to enroll approximately 210 healthy adult volunteers at KPWHRI, Emory University in Atlanta, and 2 other clinical research sites in the United States that are part of the NIAID-funded Infectious Diseases Clinical Research Consortium.
“The B.1.351 SARS-CoV-2 variant, first identified in the Republic of South Africa, has been detected in at least 9 states in the United States,” said NIAID Director Anthony S. Fauci, MD. “Preliminary data show that the COVID-19 vaccines currently available in the United States should provide an adequate degree of protection against SARS-CoV-2 variants. However, out of an abundance of caution, NIAID has continued its partnership with Moderna to evaluate this variant vaccine candidate should there be a need for an updated vaccine.”
Investigators from NIAID and Moderna previously co-developed a COVID-19 vaccine that received Emergency Use Authorization in December from the U.S. Food and Drug Administration for adults 18 years of age and older. Moderna’s new vaccine candidate differs from the currently authorized Moderna vaccine in that it incorporates aspects of the variant that was first seen in South Africa.
“In a remarkably short period of time, scientists at Moderna and NIAID have created a new vaccine based on their previous vaccine, which is now being given to millions of people worldwide,” said Lisa A. Jackson, MD, MPH, a KPWHRI senior investigator who is overseeing the trial at KPWHRI. “As we advance this study in the coming weeks, it’s important to continue wearing masks, avoiding crowds, washing hands, and getting vaccinated when you can.”
The trial will enroll adults who already have received the original Moderna vaccine, as well as adults who have not received any COVID-19 vaccine.
About 60 volunteers who previously received the first Moderna coronavirus vaccine as a participant in NIAID’s phase 1 trial are expected to enroll in the new trial. Roughly 1 year ago these volunteers received 2 vaccinations of mRNA-1273 — the name of the vaccine — 28 days apart at varying doses. As part of the new variant vaccine trial, these volunteers will be randomized to receive either a single booster vaccination of the new vaccine candidate or a single vaccination of the original vaccine and 1 dose of the new candidate.
Suzanne Uvelli-Spencer, MD, a 76-year-old retired physician, is one of the volunteers who received her shot at KPWHRI the morning of April 1. “I find it really exciting that Kaiser Permanente has this study — it’s a great honor,” said Dr. Uvelli-Spencer, who had practiced for several decades in the Seattle area.
She participated in the earlier phase 1 clinical trial a year ago. “I would have preferred being on the front line [as a physician], so doing this is the best I can do,” she said a few minutes after receiving the latest shot.
In addition to volunteers who were part of Moderna’s original phase 1 trial, the new one for the variant will enroll another 150 volunteers ages 18 through 55 years who have not received any COVID-19 vaccine, have no known history of COVID-19, and do not have health conditions that are associated with an increased risk of severe illness from COVID-19. These volunteers will be randomly assigned to 1 of 8 cohorts, with each cohort receiving different doses of the vaccines under different schedules.
Participants will be closely monitored for safety and will be asked to return to the study clinic for multiple follow-up visits between vaccinations and for additional visits during the year after their last vaccination. Scientists will measure the immune response against circulating strains of SARS-CoV-2, including the variant first detected in South Africa.
An independent safety monitoring committee will oversee the trial by regularly reviewing safety reports.
In addition to KPWHRI and Emory, the phase 1 clinical trial will enroll participants at Vanderbilt University Medical Center in Nashville and Cincinnati Children’s Hospital Medical Center.
Investigators anticipate the trial will be fully enrolled by the end of April 2021. The results of this trial will inform further evaluation of vaccine variant strategies, should an updated vaccine be required.
Individuals interested in being considered for COVID-19 vaccine clinical trials at KPWHRI may add their names to the KPWHRI vaccine registry. To learn more, please visit https://corona.kpwashingtonresearch.org/.
This story is based, in part, on an NIAAD news release that provides additional detail about the trial.
By Jonathan Rabinovitz
1-year anniversary
Looking back: Our COVID-19 vaccine trial at 1 year
Dr. Rita Mangione-Smith reflects on the year since KPWHRI launched the world's first clinical trial for a COVID-19 vaccine.
Vaccine research

COVID-19 vaccine trial registry open for volunteers
KPWHRI seeks Seattle-area residents for studies of COVID-19 vaccines.
news

Janssen phase 3 COVID-19 vaccine trial to begin at KPWHRI
The institute is participating in NIAID tests of Moderna and Johnson & Johnson vaccines.
Research
COVID-19 pandemic research at KPWHRI
Having long tracked infectious diseases and tested vaccines, KPWHRI now focuses on the novel coronavirus.



