Health Informatics
Research overview
Research on health informatics at Kaiser Permanente Washington focuses on developing and using health information technology (IT) to transform health care delivery. By testing new paradigms of care that provide more opportunities to engage patients, this research is supplying valuable evidence that is helping shape federal policy and guiding innovative redesign of health care.
“We’re working to understand how to make health IT practical so patients and care teams find it useful and engaging,” explained Kaiser Permanente Washington Health Research Institute (KPWHRI) Senior Investigator James Ralston, MD, MPH. “We want to find ways to use information technologies to support patients and providers together, both inside and outside the office.”
Integral to this support is designing technologies that are user-friendly and meet the needs of both patients and providers. By applying human-centered methods that focus on needs, use, and usability, KPWHRI researchers inform the design of health IT with direct participation from users.
Groundbreaking methodological work by KPWHRI health informatics researchers includes developing natural language processing (NLP) to analyze text such as notes and written reports in electronic health records (EHRs). Assistant Investigator David Carrell, PhD, leads in the area of using NLP and machine learning to identify patient phenotypes, or specific health characteristics such as possible heart disease, risk of opioid overdose, or suggestion of colon cancer. This information can assist researchers in studying how genetics and other factors influence disease.
Other examples of KPWHRI health informatics research include projects using EHRs and secure electronic communications such as:
- using a patient-shared EHR to improve care for chronic illnesses such as depression, diabetes, hypertension, and heart disease;
- understanding the effects of technologies such as OpenNotes, which gives patients access to notes that their doctors write during office visits;
- understanding and addressing differences in patient use of online health care services that could lead to disparities in care;
- testing NLP to target mentions of specific words and phrases in EHRs to supplement or replace skilled chart abstraction—providing faster access to “big data” and actionable information about patients who may need follow up.
Examples of KPWHRI research in mobile health (mHealth) and user-centered design include:
- evaluating mHealth smartphone tools: 1) to improve primary care for alcohol use disorders, 2) to support patients after bariatric surgery, and 3) to change smoking behavior;
- the VITAL and Seeing Priorities studies to apply user-centered processes to learn how health care providers can elicit and honor what is most important to patients living with multiple chronic health conditions;
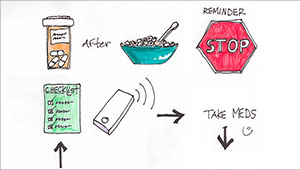
- the REMIND project applying user-centered methods to redesign clinical reminders and notifications for patients with chronic and preventive health care needs;
- the landmark Electronic Communications and Blood Pressure (eBP) study of home blood pressure monitoring and web-based care to increase hypertension control without office visits.
“Our studies on using health IT to improve care are showing that we can achieve better outcomes when we shift care from the doctor’s office to where people live: in their homes—and online,” said Senior Investigator Beverly B. Green, MD, MPH.
Recent publications on Health Informatics
Anthony MS, Armstrong MA, Getahun D, Scholes D, Gatz J, Schulze-Rath R, Postlethwaite D, Merchant M, Alabaster AL, Chillemi G, Raine-Bennett T, Xie F, Chiu VY, Im TM, Takhar HS, Fassett M, Grafton J, Cronkite D, Ichikawa L, Reed SD, Hui SL, Ritchey ME, Saltus CW, Andrews EB, Rothman KJ, Asiimwe A, Lynen R, Schoendorf J Identification and validation of uterine perforation, intrauterine device expulsion, and breastfeeding in four health care systems with electronic health records 2019 Jan;11:635-643. doi: 10.2147/CLEP.S201044. Epub 2019-07-23. PubMed
Powell BJ, Fernandez ME, Williams NJ, Aarons GA, Beidas RS, Lewis CC, McHugh SM, Weiner BJ Enhancing the Impact of Implementation Strategies in Healthcare: A Research Agenda 2019 Jan;7:3. doi: 10.3389/fpubh.2019.00003. Epub 2019-01-22. PubMed
Liao P, Dempsey W, Sarker H, Hossain SM, Al'absi M, Klasnja P, Murphy S Just-in-Time but Not Too Much: Determining Treatment Timing in Mobile Health 2018 Dec;2(4). doi: 10.1145/3287057. PubMed
Mosley JD, Benson MD, Smith JG, Melander O, Ngo D, Shaffer CM, Ferguson JF, Herzig MS, McCarty CA, Chute CG, Jarvik GP, Gordon AS, Palmer MR, Crosslin DR, Larson EB, Carrell DS, Kullo IJ, Pacheco JA, Peissig PL, Brilliant MH, Kitchner TE, Linneman JG, Namjou B, Williams MS, Ritchie MD, Borthwick KM, Kiryluk K, Mentch FD, Sleiman PM, Karlson EW, Verma SS, Zhu Y, Vasan RS, Yang Q, Denny JC, Roden DM, Gerszten RE, Wang TJ Probing the Virtual Proteome to Identify Novel Disease Biomarkers 2018 Nov 27;138(24):2787-2797. doi: 10.1161/CIRCULATIONAHA.118.036063. PubMed
Stanick CF, Halko HM, Dorsey CN, Weiner BJ, Powell BJ, Palinkas LA, Lewis CC Operationalizing the 'pragmatic' measures construct using a stakeholder feedback and a multi-method approach 2018 Nov 22;18(1):882. doi: 10.1186/s12913-018-3709-2. Epub 2018-11-22. PubMed
Researchers in Health Informatics
 Claire Allen, MPHManager, Collaborative Science |
 Katharine A. Bradley, MD, MPHSenior Investigator |
 Yates Coley, PhDAssociate Biostatistics Investigator |
 Beverly B. Green, MD, MPHSenior Investigator |
 Annie Hoopes, MD, MPHAssistant Investigator |
 Paula Lozano, MD, MPHSenior Investigator; Director, ACT Center |
 James D. Ralston, MD, MPHSenior Investigator |
 Brian D. Williamson, PhDAssociate Biostatistics Investigator |